Abstract
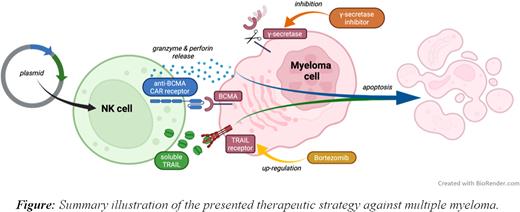
Introduction Multiple myeloma (MM) has always harbored the reputation of being incurable. However, the patients' quality of life has significantly improved over the last decades, thanks to the therapeutic contribution of allogeneic stem cell transplantation, proteasome inhibitors, immunomodulators, and monoclonal antibodies. Recently, chimeric antigen receptor (CAR) T cells targeting the B-cell maturation antigen (BCMA) have become a promising treatment strategy [1]. Due to the inherent risks associated with allogenic T cells, such as cytokine release syndrome [2] or graft-versus-host disease [3], we explored the possibility of expressing the BCMA CAR in Natural killer cells (NKs) - innate immune cells with strong cytolytic activity against cancer and minimum side effect [4]. Since we believe combinatorial therapy is the way to obtain the best therapeutical results [5], we co-expressed the BCMA CAR in the NKs with the soluble form of the tumor necrosis factor-related apoptosis-inducing ligand (sTRAIL), driving the apoptosis of the cancer cells overexpressing TRAIL receptor (TRAIL-R). In addition, anti-BCMA-CAR NKs expressing sTRAIL have the potential for a strong synergism with Bortezomib (BZ), a proteasome inhibitor commonly used in MM treatment, and γ-secretase inhibitors (GSI), which prevents the shedding of the BCMA from the MM cell surface.Methods NK-92 cell line was used as NKs source. Anti-BCMA CAR and sTRAIL coding sequences, alongside the eGFP and Nluc biomarkers, were cloned into a single plasmid interspaced with self-cleaving peptides and then integrated into the NKs genome by the action of the PiggyBac transposon system upon electroporation (NeonTM system). NKs cytotoxicity was tested on RPMI 8226, MM1.S, U266, and KMS-12-BM cell lines (luciferase-based method, 24h assay) and on isolated primary MM cells (Calcein AM staining, 4h assay). Phenotypic analyses were evaluated by flow cytometry (BD FACSAriaTM III).
Results We successfully integrated the stable expression of the transgene in NK-92 cells (CAR-NK-92-TRAIL), with more than 90% NKs positive. In a 24h coculture assay with a panel of MM cell lines, CAR-NK-92-TRAIL cells demonstrated a robust cytotoxic effect - significantly higher in each cell line compared to unmodified NKs (+ 23-63%). To evaluate potential combinatorial strategies, we studied the expression of TRAIL-R in MM cells after treatment with BZ and their expression of BCMA after GSI treatment. We observed a post-treatment increase in both targets, confirming the potential synergy between CAR-NK-92-TRAIL and BZ/GSI. Synergism was verified in cytotoxic assay with MM1.S cell line, where these drugs significantly increased (approx. 5 times) MM cell lysis in combination with CAR-NK92-TRAIL. Finally, to further validate the translational relevancy of our study, the CAR-NK-92-TRAIL cells were tested against primary MM cells. Again, we observed strong cytotoxicity against all samples tested (5:1 (E:T) ratio). Compared to unmodified NKs, the decrease in primary MM cell viability was significantly greater (approx. 15%).
Conclusion Our findings demonstrate the therapeutic efficacy of NKs expressing anti-BCMA CAR and sTRAIL against MM. In addition, we propose their combination with BZ and GSI, which enhance the expression of the therapeutic targets, resulting in a synergistic antitumor effect. Our results fully support and justify the continued development of allogeneic CAR-based products for the treatment of MM.
Disclosures
Hájek:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Consultant or advisory relationship, Research Funding; Celgene: Honoraria, Other: Consultant or advisory relationship, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Consultant or advisory relationship, Research Funding; AbbVie: Honoraria, Other: Consultant or advisory relationship; PharmaMar: Honoraria, Other: Consultant or advisory relationship; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Consultant or advisory relationship, Research Funding; Novartis: Other: Consultant or advisory relationship, Research Funding; Bristol Myers Squibb: Honoraria, Other: Consultant or advisory relationship, Research Funding. Bago:Bayer: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal